SKYCLARYS is the
First and Only FDA-Approved Treatment for Friedreich Ataxia (FA) in Patients 16 Years and Older1,2
Not an actual patient.
MOXIe trial
SKYCLARYS was studied in the MOXIe trial, the largest FA study of its kind1,2
Designed in consultation with clinical specialists, patient advocacy groups, and the FDA. The MOXIe trial was an international, double-blind, randomized, placebo-controlled, multicenter, registrational phase 2 trial. A total of 103 patients were randomized in a ratio of 1:1 (the All Randomized Population) to receive SKYCLARYS 150 mg once daily (n=51) or placebo (n=52).
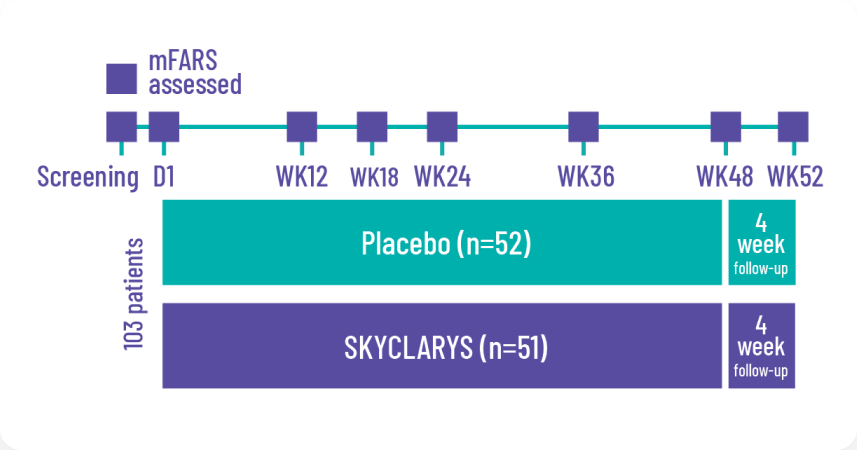
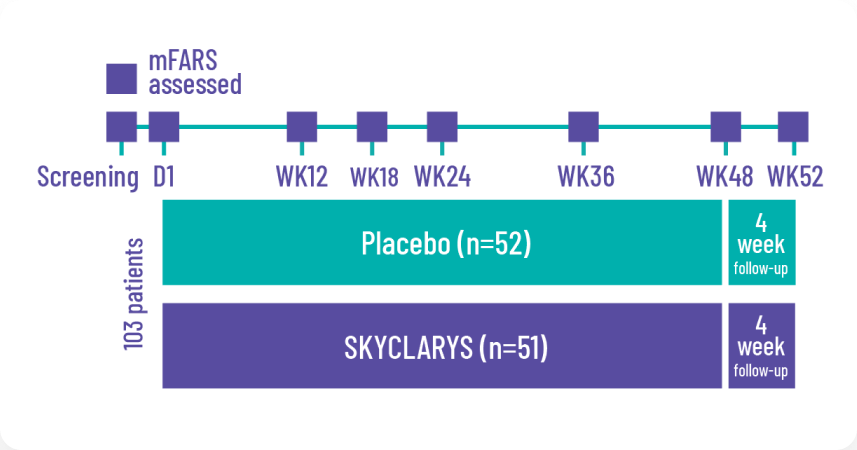
The prespecified primary analysis
was the change from baseline in the mFARS score compared with placebo at Week 48 in the Full Analysis Population of patients without pes cavus (n=82).2
SKYCLARYS slowed FA disease progression in the pivotal MOXIe trial1
Treatment with SKYCLARYS resulted in less physical impairment relative to placebo at Week 48.
ADVERSE REACTIONS
- Adverse reactions reported in 10% or more of patients and greater than placebo were elevated liver enzymes (AST/ALT), headache, nausea, abdominal pain, fatigue, diarrhea, musculoskeletal pain, oropharyngeal pain, influenza, vomiting, muscle spasms, back pain, decreased appetite, and rash1
Results from patient subgroups numerically favored SKYCLARYS over placebo1,2:
- Age (±18 years)
- Sex
- GAA repeat length (≥675)
- Ambulatory status
- Presence of pes cavus
All 4 components of the mFARS assessment numerically favored SKYCLARYS over placebo.2
Bulbar function
Lower limb coordination
Upper limb coordination
Upright stability
In adolescent patients aged 16 to 18 years treated with SKYCLARYS (n=20) a group difference of -4.16 points versus placebo was observed at Week 48.2
The MOXIe trial was not powered to detect a statistically significant difference among subgroups.2
Results of a 3-Year Post Hoc Propensity-Matched Analysis
These exploratory analyses should be interpreted cautiously given the limitations of data collected outside of a controlled study, which may be subject to confounding.
Lower mFARS scores were observed in patients treated with SKYCLARYS after 3 years relative to a matched set of patients from a natural history study.1
Propensity matching is a method of comparing patients from a clinical trial with an external control by identifying comparable prognostic characteristics. It is informative in cases where a very long follow-up period is required to assess outcomes or when it is difficult to perform randomized controlled trials, such as in certain special patient populations.
An ongoing MOXIe open-label (OLE) assesses long-term safety and tolerability of omaveloxolone in patients with FA who completed MOXIe Part 1 or Part 2 (n=136). A post hoc propensity-matched analysis compared patients in the MOXIe OLE with patients who were not treated with SKYCLARYS who participated in a natural history study, the Friedreich Ataxia Clinical Outcome Measures Study (FA-COMS).
Patient characteristics in the propensity-matched analysis:
FA-COMS is a large, robust FA natural history study and has enrolled more than 1350 patients who have been followed for up to 15 years.
mFARS
What is mFARS?2,6
The mFARS is a clinically validated neurological assessment that provides a detailed evaluation of a patient’s status and is generally accepted as a clinical trial endpoint due to its correlation with disease progression. mFARS is not required to be used in the clinic in order to treat with SKYCLARYS.
mFARS range is 0-93 points
A higher score is associated with more severe disease.2,6
The mFARS is made up of 4 sections focused on functional abilities6:
Ability to complete many activities of daily living, such as getting dressed and eating
Predictor of time to loss of ambulation; the average score where loss of ambulation occurs is 65
Closely correlated with upright stability in predicting the likely rate of disease progression
Ability to speak clearly
While there is variability in progression, a patient’s mFARS score will worsen (increase) by about 2 points per year on average7
Patients with a younger age of onset may progress faster.
References:
1. Skyclarys. Prescribing Information. Biogen; 2024. 2. Lynch DR, Chin MP, Delatycki MB, et al. Safety and efficacy of omaveloxolone in Friedreich ataxia (MOXIe study). Ann Neurol. 2021;89(2):212-225. doi:10.1002/ana.25934 3. Parkinson MH, Boesch S, Nachbauer W, Mariotti C, Giunti P. Clinical features of Friedreich’s ataxia: classical and atypical phenotypes. J Neurochem. 2013;126(suppl 1):103-117. doi:10.1111/jnc.12317 4. Schulz JB, Boesch S, Bürk K, et al. Diagnosis and treatment of Friedreich ataxia: a European perspective. Nat Rev Neurol. 2009;5(4):222-234. doi:10.1038/nrneurol.2009.26 5. Rummey C, Farmer JM, Lynch DR. Predictors of loss of ambulation in Friedreich’s ataxia. eClinicalMedicine. 2020;18:1-9. doi:10.1016/j.eclinm.2019.11.006 6. Rummey C, Corben LA, Delatycki MB, et al. Psychometric properties of the Friedreich Ataxia Rating Scale. Neurol Genet. 2019;5(6):371. doi:10.1212/NXG.0000000000000371 7. Patel M, Isaacs CJ, Seyer L, et al. Progression of Friedreich ataxia: quantitative characterization over 5 years. Ann Clin Transl Neurol. 2016;3(9):684-694. doi:10.1002/acn3.332 8. Lynch DR, Chin MP, Boesch S, et al. Efficacy of omaveloxolone in Friedreich’s ataxia: delayed-start analysis of the MOXIe extension. Mov Disord. 2023;38(2):313-320. doi:10.1002/mds.29286 9. Galea CA, Huq A, Lockhart PJ, et al. Compound heterozygous FXN mutations and clinical outcome in Friedreich ataxia. Ann Neurol. 2016;79(3):485-495. doi:10.1002/ana.24595 10. Fogel BL, Perlman S. Clinical features and molecular genetics of autosomal recessive cerebellar ataxias. Lancet Neurol. 2007;6(3):245-257. 11. Wallace SE, Bird TD. Molecular genetic testing for hereditary ataxia: what every neurologist should know. Neurol Clin Pract. 2018;8(1):27-32. doi:10.1212/CPJ.0000000000000421 12. Data on file. Reata Pharmaceuticals, Inc.; 2022. 13. Lynch DR, Goldsberry A, Rummey C, et al. Propensity matched comparison of omaveloxolone treatment to Friedreich ataxia natural history data. Ann Clin Transl Neurol. 2024;11(1):4-16. doi:10.1002/acn3.51897
14. Beaulieu-Jones BK, Finlayson SG, Yuan W, et al. Examining the use of real-world evidence in the regulatory process. Clin Pharmacol Ther. 2020;107(4):843-852. doi:10.1002/cpt.1658
